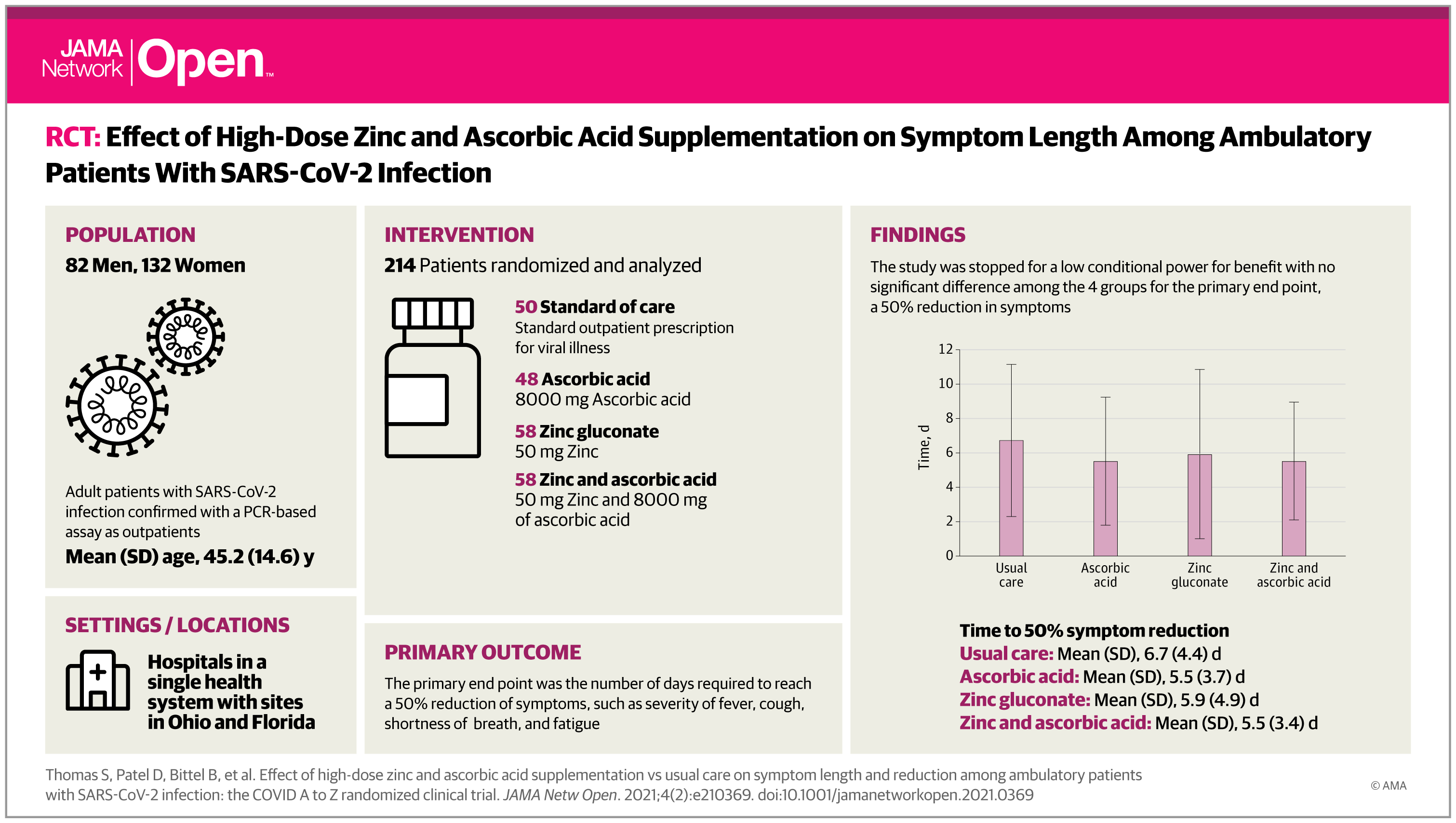
Prevention - Colchicine reduces risk of COVID-19 complications
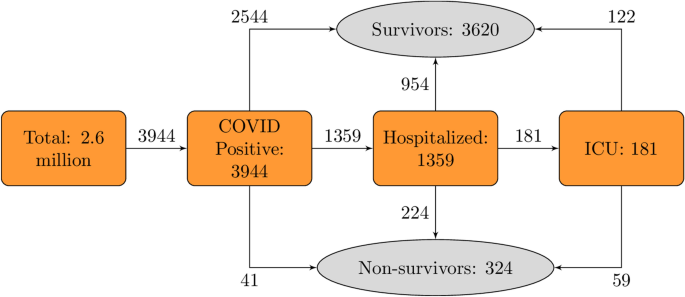
Colchicine has reduced by 21% the risk of death or hospitalizations in patients with COVID-19 compared to placebo. This result obtained for the global study population of 4488 patients approached statistical significance. The analysis of the 4159 patients in whom the diagnosis of COVID-19 was proven by a naso-pharyngeal PCR test has shown that the use of colchicine was associated with statistically significant reductions in the risk of death or hospitalization compared to placebo. In these patients with a proven diagnosis of COVID-19, colchicine reduced hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%. This major scientific discovery makes colchicine the world’s first oral drug that could be used to treat non-hospitalized patients with COVID-19.
 www.colcorona.net
www.colcorona.net
The addition of colchicine to standard treatment reduces the duration of supplemental oxygen therapy and time to hospital discharge among patients with moderate-to-severe COVID-19, suggest findings from a randomized controlled trial.
In the RECOVERY trial, not shown to have a benefit. In adults hospitalised with COVID-19, colchicine was not associated with reductions in 28-day mortality, duration of hospital stay, or risk of progressing to invasive mechanical ventilation or death.

Colchicine has reduced by 21% the risk of death or hospitalizations in patients with COVID-19 compared to placebo. This result obtained for the global study population of 4488 patients approached statistical significance. The analysis of the 4159 patients in whom the diagnosis of COVID-19 was proven by a naso-pharyngeal PCR test has shown that the use of colchicine was associated with statistically significant reductions in the risk of death or hospitalization compared to placebo. In these patients with a proven diagnosis of COVID-19, colchicine reduced hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%. This major scientific discovery makes colchicine the world’s first oral drug that could be used to treat non-hospitalized patients with COVID-19.
COVID - 19 - Clinical Trial | ColCorona
On January 22, 2021, the Montreal Heart Institute announced positive results from COLCORONA trial.The COLCORONA study manuscript is now available in the public domain.
The addition of colchicine to standard treatment reduces the duration of supplemental oxygen therapy and time to hospital discharge among patients with moderate-to-severe COVID-19, suggest findings from a randomized controlled trial.
In the RECOVERY trial, not shown to have a benefit. In adults hospitalised with COVID-19, colchicine was not associated with reductions in 28-day mortality, duration of hospital stay, or risk of progressing to invasive mechanical ventilation or death.

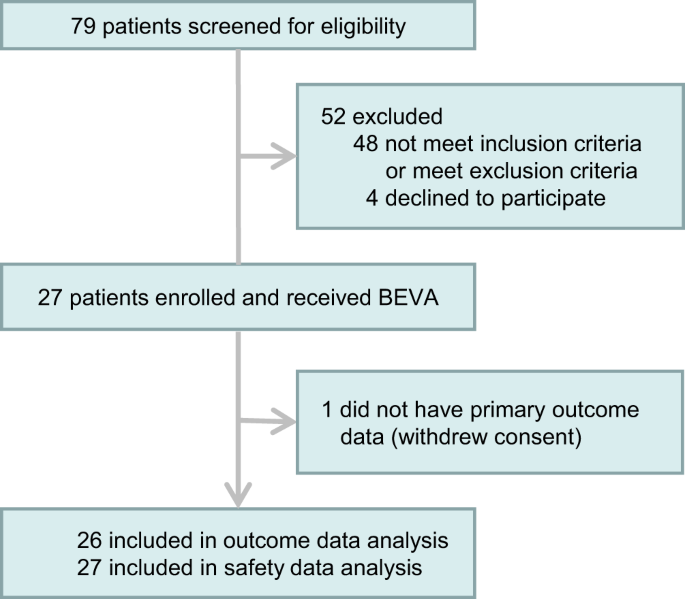
Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
In adults hospitalised with COVID-19, colchicine was not associated with reductions in 28-day mortality, duration of hospital stay, or risk of progressing to invasive mechanical ventilation or death.
www.thelancet.com
Last edited: