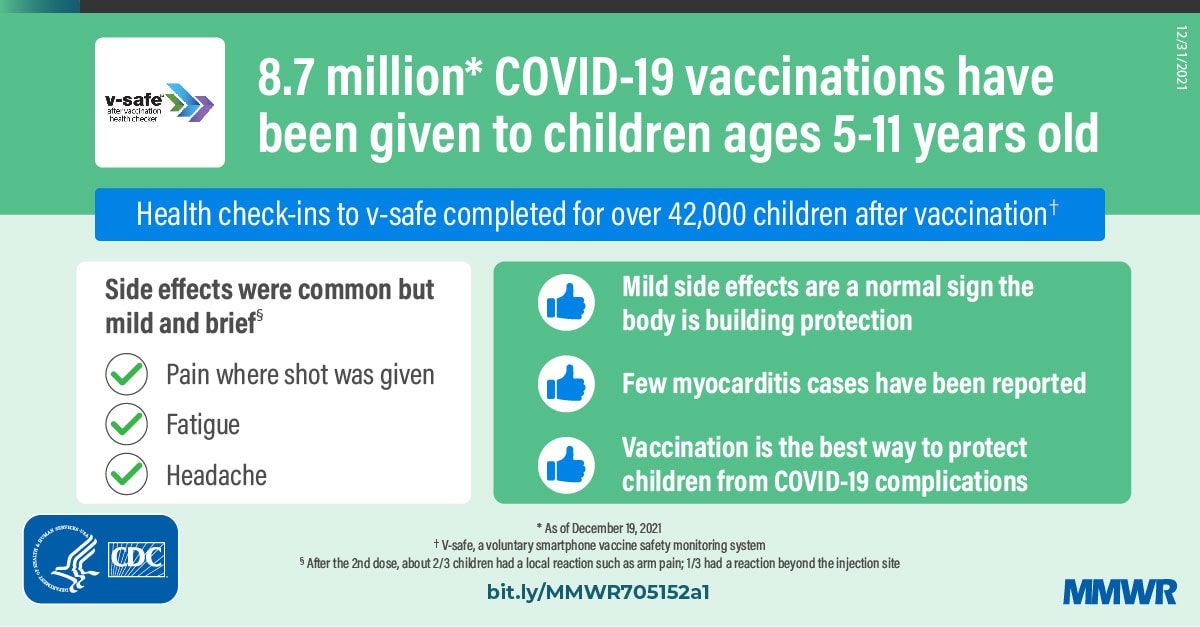
Rapid Antigen Testing
These assays correctly identify a SARS-CoV-2 infection in 72 percent of people with symptoms and 58 percent of people without them. The tests detect an average of 78 percent of cases in the first week of symptoms but only 51 percent during the second week. A negative test can ease anxieties, at least for the time being—but people with symptoms should still follow up with a more accurate test. Antigen tests, which detect pieces of the virus’s proteins, are considered less sensitive to low amounts of virus than the more accurate PCR tests.
98% pickup rate if test done every 3 days according to one small study of 43 people. Longitudinal Assessment of Diagnostic Test Performance Over the Course of Acute SARS-CoV-2 Infection
Infected people with low levels of virus in their nose (usually described as a low viral load) typically do not spread the virus.
 consultqd.clevelandclinic.org
consultqd.clevelandclinic.org
The CDC recommends that symptomatic people with a negative antigen test should follow up with a PCR test within 48 hours.

These assays correctly identify a SARS-CoV-2 infection in 72 percent of people with symptoms and 58 percent of people without them. The tests detect an average of 78 percent of cases in the first week of symptoms but only 51 percent during the second week. A negative test can ease anxieties, at least for the time being—but people with symptoms should still follow up with a more accurate test. Antigen tests, which detect pieces of the virus’s proteins, are considered less sensitive to low amounts of virus than the more accurate PCR tests.
98% pickup rate if test done every 3 days according to one small study of 43 people. Longitudinal Assessment of Diagnostic Test Performance Over the Course of Acute SARS-CoV-2 Infection
Infected people with low levels of virus in their nose (usually described as a low viral load) typically do not spread the virus.

What Viral Load Can Tell Us About the Transmission Potential of COVID-19 in Healthcare Workers
A new Cleveland Clinic-led study examines the distribution of viral load in healthcare providers who tested positive for COVID-19. In the majority of cases, the findings show, the risk of transmission is relatively low after seven to 10 days following the onset of symptoms.
The CDC recommends that symptomatic people with a negative antigen test should follow up with a PCR test within 48 hours.